Why Would We Continue to Draw Cardiac Biomarkers Every 8 Hours X 3
Recent advances in heart failure (HF) biomarker studies suggest promise from markers enhancing traditional method of assessing affected patients. B-type natriuretic peptide (BNP) and its biologically inert, amino-terminal pro-peptide counterpart (NT-proBNP)1,2 have quickly become an essential component in the diagnosis and determining prognosis in HF. With a large number of biomarkers now or soon to be available, an understanding of the role that biomarkers may play in HF care is necessary.3
Overview of Biomarkers
Despite significant overlaps, biomarkers can be loosely arranged into the following categories: 1) myocardial stress/injury, 2) neurohormonal activation, 3) remodeling and 4) comorbidities (Table 1). There are far too many to consider in a short summary; thus, only the most clinically relevant biomarkers will be discussed.
Table 1: Biomarkers in Heart Failure
| Myocardial Insult
| Neurohormonal Activation
|
| Remodeling
| Comorbidities
|
1. Myocardial Stress/Injury
BNP and NT-proBNP are considered the benchmarks against which other biomarkers are compared. Biomarkers of myocardial necrosis and oxidative stress are also included in this category.
BNP and NT-proBNP
The most potent inducer of BNP gene transcription is left ventricular (LV) wall stretch from increased pressure or volume. A prohormone (proBNP) is cleaved to BNP and NT-proBNP, resulting in a serologic evidence of BNP, NT-proBNP, and proBNP. Conventional assays for BNP detect proBNP and BNP, as well as various degraded fragments of BNP, while NT-proBNP assays detect NT-proBNP and proBNP.4
Stimulation of the natriuretic peptide receptor by BNP triggers natriuresis, diuresis, vasodilation, inhibition of renin and aldosterone and inhibition of fibrosis. BNP is removed from circulation by a receptor-mediated mechanism as well as degradation by neutral endopeptidases such as neprilysin (the same enzyme inhibited by the novel drug, LCZ696).5 Importantly, administration of LCZ696 significantly increases BNP (but not NT-proBNP) values as part of the effect of the drug. Therefore, interpretation of BNP in a patient taking LCZ696 will be challenging, if not impossible. Beyond these modes of clearance, both BNP and NT-proBNP are passively cleared by a number of organs including kidneys. The half-life of BNP is significantly shorter than that of NT-proBNP (approximately 20 vs. 60-120 minutes).
Diagnostic evaluation of acute HF
Normally, circulating BNP and NT-proBNP levels are quite low, but in the setting of HF, their concentrations rise dramatically (Table 2). The Breathing Not Properly Multinational Study1 demonstrated that BNP values >100 pg/mL diagnosed acute HF with high accuracy at 85% and strongly predicted of HF, outperforming clinical criteria. Similar findings were seen for NT-proBNP in the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study2 in which elevated NT-proBNP concentrations were the strongest predictor of HF compared with traditional assessment. A single NT-proBNP cutoff of 900 pg/mL provided similar diagnostic performance as BNP of 100 pg/mL, but age-stratified cutoff points for NT-proBNP (≥450 for ages <50 years, ≥900 for 50-75 years and ≥1800 pg/mL for >75 years) performed the best.6 The best diagnostic approach for use of either BNP or NT-proBNP is to support clinical judgment, rather than replace it; indeed, both biomarkers are particularly useful when diagnostic indecision is present. Beyond their ability to diagnose HF when markedly elevated, both BNP and NT-proBNP are also useful to exclude the diagnosis when very low.
Table 2: Natriuretic Peptide Cutoff Points
| Cut-off Value | Sensitivity | Specificity | PPV | NPV | ||
| Acute Dyspnea | To exclude acute HF | |||||
| BNP | <30-50 pg/mL | 97% | 62% | 71% | 96% | |
| NT-proBNP | <300 pg/mL | 99% | 68% | 62% | 99% | |
| To identify acute HF | ||||||
| Single cut-off point | ||||||
| BNP | <100 pg/mL | 90% | 76% | 79% | 89% | |
| NT-proBNP | <900 pg/mL | 90% | 85% | 76% | 94% | |
| Multiple cut-point | ||||||
| BNP | < 100 pg/mL to exclude | 90% | 73% | 75% | 90% | |
| 100-400 pg/mL, "grey zone" | * | * | * | * | ||
| > 400 pg/mL, to rule in | 63% | 91% | 86% | 74% | ||
| NT-proBNP | <450 pg/mL for age <50 yr | 90% | 84% | 88% | 66% | |
| Outpatient Screening | BNP | 20 pg/mL (asymptomatic) | * | * | * | 96% |
| or 40 pg/mL (symptomatic) | * | * | * | |||
| NT-proBNP | <125 pg/mL for age <75 yr | * | * | * | 98% | |
| <450 pg/mL for age ≥75 yr | * | * | * | 91% | ||
| or | ||||||
| <50 pg/mL for age <50 yr | * | * | * | 98% | ||
| <75 pg/mL for age 50-75 yr | * | * | * | 98% | ||
| < 250 pg/mL for age > 75 yr | * | * | * | 93% |
Modified from Kim H-N, Januzzi JL, Jr. Natriuretic peptide testing in heart failure. Circulation 2011;123:2015-19.
Caveats of natriuretic peptides
Beyond "HF," a number of cardiopulmonary disorders are associated with elevated BNP or NT-proBNP values: acute coronary syndrome, myocarditis, valvular heart disease, hypertrophic cardiomyopathy, cardiotoxic drugs, atrial fibrillation or flutter and right ventricular dysfunction in the setting of significant pulmonary disease (pulmonary hypertension, pulmonary embolism). Other conditions that are associated with higher BNP or NT-proBNP levels may be related to comorbidities (advanced age, renal dysfunction, stroke and critical illness) and high output states. Lastly, as noted above, use of neprilysin inhibitors (such as that within LCZ696) leads to pharmacologic 'raising' of BNP values; notably these drugs do not affect NT-proBNP levels. When neprilysin inhibition becomes more widespread the ability to interpret BNP will be substantially challenged in those patients so treated.
Some conditions are associated with lower than expected natriuretic peptide levels: HF with preserved ejection fraction (HFpEF) compared with HF with reduced ejection fraction (HFrEF) and obesity. Obesity has been associated with lower than expected BNP or NT-proBNP values, likely due to the suppression of natriuretic peptide synthesis or release. However elevated BNP and NT-proBNP can still diagnose acute HF regardless of body-mass index accurately, but with a lower sensitivity.7 As a matter of fact, the age-stratified cutoff points for NT-proBNP have a sensitivity of 86%, specificity of 90% with a positive predictive value of 85% and negative predictive value of 95% in obese patients.8 With respect to HFpEF, the same cutoff points for BNP or NT-proBNP can be utilized to diagnose HF with the understanding that the sensitivity is somewhat decreased.9,10
Prognosis and treatment of HF
BNP and NT-proBNP are strong predictors of clinical outcomes in all stages of HF. In the Acute Decompensated Heart Failure National Registry (ADHERE) registry,11 admission BNP values for acute HF decompensation in the highest quartile (BNP ≥1730 pg/mL) was associated with 2.23-fold increase in in-hospital mortality compared with BNP in the lowest quartile (<430 pg/mL), even after adjustment for potential confounders and regardless of EF. Likewise, NT-proBNP concentrations are strongly predictive of short and long-term clinical outcomes; admission NT-proBNP >986 pg/mL was associated with almost three-fold increase in one-year mortality (adjusted hazard ratio [HR] 2.88).6,12 Importantly, both absolute natriuretic peptide concentrations at discharge as well as percent change from admission to discharge provide substantially greater information regarding risk for subsequent mortality and/or rehospitalization than admission values. In the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) trial,13 the discharge (rather than admission) log-transformed BNP was the most important predictor of one-year mortality (HR of 1.34) and one-year death or rehospitalization (HR 1.15). This suggests a strategy of admission/discharge sampling is justifiable.
Serial measurement in chronic HF patients also strongly predict prognosis. In the Valsartan Heart Failure Trial (Val-HeFT) study,14 a four-month log-transformed continuous NT-proBNP value added incremental prognostic value to a baseline measurement in a multivariable model to predict mortality (HR = 1.99). A direction of change in NT-proBNP values over time closely reflected changes in prognosis. Patients with baseline NT-proBNP increase over four months from below the cutoff value of 1,078 pg/mL to above the cutoff value was associated with a 1.7-fold increase in risk of mortality (HR = 1.70), compared with NT-proBNP below the cutoff value throughout, and similar in risk to those whose NT-proBNP values remained high throughout (HR 1.88).
HF therapies that improve mortality and morbidity in HF reduce these natriuretic peptide values.15 This observation has led to the concept of biomarker-guided HF management using BNP or NT-proBNP to supplement clinical judgment. Meta-analyses suggest a 20-30% mortality reduction with biomarker-guided HF care over standard HF care and prospective studies have demonstrated that serial outpatient assessment of natriuretic peptides leads to more uptitration of HF medications and decrease in natriuretic peptides.16,17 A large randomized controlled trial, the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT) trial, will examine the merits of NT-proBNP guided care in patients with HFrEF.18
Troponins
Beyond their ability to diagnose myocardial infarction, troponin T (TnT) and I (TnI), are frequently detectable in HF.19 In the ADHERE registry,20 6.2% of patients with acute decompensated HF had an elevated troponin (as defined by conventional troponin assays as ≥1.0 μg/L for troponin I or ≥0.1μg/L for troponin T), which was associated with worse in-hospital mortality (adjusted odds ratio = 2.55). Using high sensitivity (hs) troponin assays, substantially more patients with acutely decompensated HF have abnormal concentrations.21 Elevated hsTnT was independently predictive of all-cause mortality in a multivariable model including NT-proBNP as well as ST2 (HR = 1.16). As acute decompensation of HF may be a part of the presentation of acute MI, guidelines from major societies recommend troponin measurement in all patients presenting acutely, in order to exclude or diagnose ischemia as a cause.
In patients with chronic HF, 92% of patients were found to have measurable or elevated hsTnT (detection limit ≤0.001 ng/mL),22 and hsTnT >0.012 ng/mL was closely linked with poor clinical outcomes (adjusted HR = 2.08 for mortality and adjusted HR = 1.55 for first HF hospitalization). Serial measurement may add to a baseline value; in a large cohort of chronic HF patients,23 increase in hsTnT over three or four months was strongly predictive of all-cause mortality (HR 1.88 after adjustment for traditional risk factors, baseline hsTnT and baseline NT-proBNP), but improvement in prediction over baseline measurement was only modest. Unfortunately, no therapies have been yet identified to reduce the development or rise of hsTn in patients with HF. Elevated TnT ≥0.035μg/L is also used for staging of cardiac AL amyloidosis and is closely associated with poor prognosis.24
2. Neurohormonal Activation
Cardiac injury leads to the activation of a number of biologically active proteins that attempt to compensate for reduced myocardial function. However, prolonged activation often leads to maladaptive effects and further progression of HF.
MR-proADM and Copeptin
One of the first responses to cardiac dysfunction is the activation of the sympathetic nervous system. Mid-regional proadrenomedullin (MR-proADM) is a precursor to a potent vasodilator with inotropic properties, adrenomedullin, originally isolated from pheochromocytoma cells. MR-proADM is elevated in patients with acute and chronic HF and is a strong predictor of clinical outcomes such as mortality and HF hospitalization, even when added to BNP or NT-proBNP.25-27 Copeptin is a stable C-terminal pro-peptide fragment of arginine vasopressin (AVP); AVP is centrally involved in the regulation of free water clearance and plasma osmolality by regulating absorption of water from the kidneys. In the Biomarkers in Acute Heart Failure (BACH) trial,28 elevated copeptin level strongly predicted mortality, and in those with hyponatremia, elevated copeptin level was more predictive, even after adjusting for NT-proBNP and traditional variables. It is tempting to speculate whether copeptin values could be used to guide therapy with vasopression receptor antagonists, however, such data are not available yet.
3. Myocardial Remodeling
Myocardial remodeling is the pivotal process leading to progressive myocardial dysfunction and risk in HF. While BNP, NT-proBNP and hsTn are all also linked to remodeling risk, other biomarkers are worth mention.
ST2
ST2 gene is strongly induced in the setting of cardiomyocyte or cardiac fibroblasts stretch. ST2 is closely involved in LV hypertrophy, fibrosis and remodeling via its interaction with interleukin (IL)-33, a protein with anti-fibrotic and anti-remodeling properties.29 Increasing ST2 concentrations (e.g. >35 ng/mL) are powerfully associated with adverse clinical outcomes in HF, and compared with BNP or NT-proBNP, ST2 is not as affected by age, renal function or BMI.
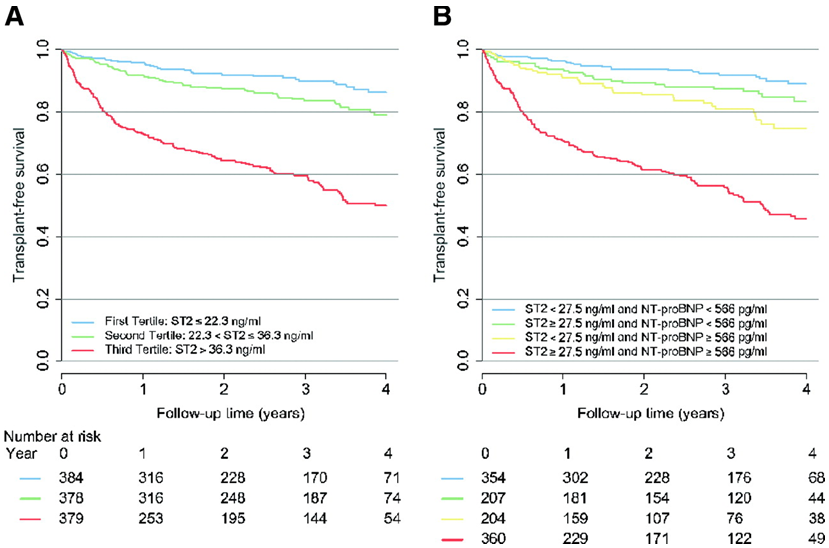
| Figure 1 | |
| ST2 and NT-proBNP Independently Predicted Outcomes in Chronic HF: Reproduced with permission from Ky B, French B, Mcloskey K, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180-7. | |
In a multivariable model that included traditional markers of risk in acute HF patients, ST2 had independent and additive prognostic information beyond NT-proBNP (HR = 9.3, Figure 1) regardless of left ventricular ejection fraction (LVEF).30 In addition, serial measurement of ST2 after acute HF therapy provided incremental information beyond a single value, and was superior to that provided by NT-proBNP.31 In a multimarker panel, ST2 value was one of the strongest predictors of mortality at 30 days and one-year in a reclassification analysis,32 and was associated with more than three-fold risk in adverse outcomes. Its ability to predict long-term outcomes appeared to be similar to that of NT-proBNP in chronic HF patients, but the best strategy was to combine the information derived from both biomarkers in a multi-marker model; adding such an approach to the traditional clinical model such as the Seattle Heart Failure Model provided incremental improvement in prediction of future outcomes. In head-to-head studies, ST2 is often the most powerful biomarker predictor of risk; for example, ST2 was recently shown to be superior to galectin-3 in patients with chronic HF.33 ST2 can change significantly over time and serial measurement of ST2 adds independent and additive information to the baseline value and traditional risk factors in chronic HF if a rise and/or fall around the cut-off of 35 ng/mL is seen.34 ST2 levels may interact with specific HF therapies. Beta-blockers, angiotensin II receptor antagonists and mineralocorticoid receptor antagonists may influence ST2 values, opening the door for the possibility of guided HF therapy using serial measurement.35-37 Large, prospective trials are needed to evaluate this further.
In lower risk populations, those with acute MI38 or in healthy community-based population,39 elevated ST2 predicted future development of HF even after adjusting for traditional and novel markers of risk.
Galectin-3
Galectin-3 is involved in the inflammatory pathway following injury and ventricular remodeling via tissue repair, myofibroblast proliferation, and fibrogenesis. Instillation of galectin-3 into the pericardium caused a significant increase in collagen deposition40 and galectin-3 genetic knockout mice were resistant to increased LV tension and progression of ventricular dysfunction. Galectin-3 is elevated in patients with acute or chronic HF41-43 and in univariable analyses is frequently associated with risk, but with adjustment for renal function or other biomarkers, galectin-3 loses its prognostic meaning in many studies. Serial measurement of galectin-3 in chronic HF patients may to add to a single measurement,44 but to date, there are no known therapies that can alter galectin-3 values.
4. Comorbidities
Comorbidities including renal dysfunction, hematologic abnormalities and liver dysfunction are important markers of poor prognosis in HF. While serum creatinine, estimated glomerular filtration rate, and blood urea nitrogen are important markers of renal function and provide prognostic information beyond traditional assessment including NT-proBNP,45 their prognostic value is weaker at milder impairment range and onset of rise in these biomarkers are often delayed after acute kidney injury (AKI). Cystatin C and β trace protein (BTP) performed better than traditional renal markers for determining prognosis in HF, presumably due to enhanced ability to gauge renal function at milder levels of abnormality.46 Several novel serum or urine biomarkers have been evaluated to date with regards to their ability to detect AKI earlier: neutrophil-gelatinase associated lipocalin (NGAL), kidney injury molecule (KIM)-1, N-acetyl β-(D)-glucosaminidase (NAG), liver-type fatty acid binding protein and IL-18. While elevated levels of NGAL are associated with poor clinical outcomes, this relationship was less impressive after adjusting for extensive variables including NT-proBNP.47 In addition, its ability to predict imminent AKI was fair (68% sensitivity and 70% specificity).48 Urinary KIM-1 and serum NAG have shown promise, but further studies are needed.
Hematologic abnormalities including anemia, iron deficiency and increased red blood cell distribution width (RDW) are rather frequent in HF patients and of importance in determining prognosis regardless of LVEF.49 Whether specific correction of such hematologic abnormalities will improve clinical outcomes in HF remains unclear.
Major Society Guidelines
The American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) HF guidelines50 have given BNP and NT-proBNP a Class I recommendation for both diagnosis and prognosis of HF (level of evidence: A). The use of these natriuretic peptides for guiding HF management received a Class IIa recommendation for chronic HF (level of evidence: B) and IIb for acute HF (level of evidence: C). TnT or TnI received Class I recommendation (level of evidence: A) for prognosis and in detection of acute myocardial infarction as the precipitant of acute HF, while biomarkers of myocardial fibrosis, soluble ST2 (ST2) and galectin-3, received Class IIb recommendations (level of evidence: B for chronic and A for acute HF).
Future Directions: Personalized "Precision" Medicine in HF
As noted, most of the value described for newer biomarkers in HF relates to prognostication. We have argued prognostic value of a biomarker is only useful if it informs specific change in clinical care;3 such change in care should also lead to improvement in prognosis that otherwise would not have occurred. This strategy is being tested in the GUIDE-IT trial for NT-proBNP. However, for all other biomarkers, it remains unclear if specific therapy changes should be made in response to an abnormal result. More information is needed. This strategy of a more personalized "precision" approach using biomarkers is already seen in oncology, but is just beginning to gain momentum in cardiology.
References
- Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161-7
- Januzzi JL, Jr., Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP. Investigation of Dyspnea in the Emergency department (PRIDE) study. Am J Cardiol 2005;95:948-54.
- Ahmad T, Fiuzat M, Pencina MJ, et al. Charting a roadmap for heart failure biomarker studies. JACC Heart Fail 2014;2:477-88.
- Niederkofler EE, Kiernan UA, O'Rear J, et al. Detection of endogenous B-type natriuretic peptide at very low concentrations in patients with heart failure. Circ Heart Fail 2008;1:258-64.
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993-1004.
- Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients The International Collaborative of NT-proBNP Study. Eur Heart J 2006;27:330-337.
- Bayes-Genis A, Lloyd-Jones DM, van Kimmenade RR, et al. Effect of body mass index on diagnostic and prognostic usefulness of amino-terminal pro-brain natriuretic peptide in patients with acute dyspnea. Arch Intern Med 2007;167:400-7.
- Kim H-N, Januzzi JL, Jr. Natriuretic peptide testing in heart failure. Circulation 2011;123:2015-19.
- Maisel AS, McCord J, Nowak RM, et al. Bedside B-Type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol 2003;41:2010-7.
- O'Donoghue M, Chen A, Baggish AL, et al. J Card Fail. 2005;11:S9-14.
- Fonarow GC, Peacock WF, Phillips CO, et al. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol 2007;49:1943-50.
- Januzzi JL, Jr., Sakhuja R, O'Donoghue M, et al. Utility of amino-terminal pro-brain natriuretic peptide testing for prediction of 1-year mortality in patients with dyspnea treated in the emergency department. Arch Intern Med 2006;166:315-20.
- Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail 2011;4:628-36.
- Masson S, Latini R, Anand IS, et al. Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial). J Am Coll Cardiol 2008;52:997-1003.
- Gaggin HK, Januzzi JL, Jr. Biomarkers and diagnostics in heart failure. Biochim biophys Acta 2013;1832:2442-50.
- Januzzi JL, Jr., Rehman SU, Mohammed AA, et al. Use of amino-terminal pro-B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011;58:1881-9.
- Shah MR, Califf RM, Nohria A, et al. The STARBRITE trial: a randomized, pilot study of B-type natriuretic peptide-guided therapy in patients with advanced heart failure. J Card Fail 2011;17:613-21.
- Braunwald E. Heart failure. JACC Heart Fail 2013;1:1-20.
- Januzzi JL, Jr., Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J 2012;33:2265-71.
- Peacock WFt, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117-26.
- Xue Y, Clopton P, Peacock WF, Maisel AS. Serial changes in high-sensitive troponin I predict outcome in patients with decompensated heart failure. Eur J Heart Fail 2011;13:37-42.
- Latini R, Masson S, Anand IS, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007;116:1242-9.
- Masson S, Anand I, Favero C, et al. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation 2012;125:280-8.
- Kumar S, Dispenzieri A, Lacy MQ, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol 2012;30:989-95.
- Maisel A, Mueller C, Nowak R, et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol 2010;55:2062-76.
- Shah RV, Truong QA, Gaggin HK, et al. Mid-regional pro-atrial natriuretic peptide and pro-adrenomedullin testing for the diagnostic and prognostic evaluation of patients with acute dyspnoea. Eur Heart J 2012;33:2197-205.
- Richards AM, Doughty R, Nicholls MG, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: prognostic utility and prediction of benefit from carvedilol in chronic ischemic left ventricular dysfunction. Australia-New Zealand Heart Failure Group. J Am Coll Cardiol 2001;37:1781-87.
- Maisel A, Xue Y, Shah K, et al. Increased 90-day mortality in patients with acute heart failure with elevated copeptin: secondary results from the Biomarkers in Acute Heart Failure (BACH) study. Circ Heart Fail 2011;4:613-20.
- Sanada S, Hakuno D, Higgins LJ, et al. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007;117:1538-49.
- Januzzi JL, Jr., Peacock WF, Maisel AS, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol 2007;50:607-13.
- Boisot S, Beede J, Isakson S, et al. Serial sampling of ST2 predicts 90-day mortality following destabilized heart failure. J Card Fail 2008;14:732-8.
- Lassus J, Gayat E, Mueller C, et al. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: the Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol 2013;168:2186-94.
- Bayes-Genis A, de Antonio M, Vila J, et al. Head-to-head comparison of 2 myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 versus galectin-3. J Am Coll Cardiol 2014;63:158-66.
- Gaggin HK, Szymonifka J, Bhardwaj A, et al. Head-to-head comparison of serial soluble ST2, growth differentiation factor-15, and highly-sensitive troponin T measurements in patients with chronic heart failure. JACC Heart Fail 2014;2:65-72.
- Gaggin HK, Motiwala S, Bhardwaj A, et al. Soluble concentrations of the interleukin receptor family member ST2 and β-blocker therapy in chronic heart failure. Circ Heart Fail 2013;6:1206-13.
- Weir RA, Miller AM, Murphy GE, et al. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol 2010;55:243-50.
- Anand IS, Rector TS, Kuskowski M, et al. Prognostic value of soluble ST2 in the Valsartan Heart Failure Trial. Circ Heart Fail 2014;7:418-26.
- Kohli P, Bonaca MP, Kakkar R, et al. Role of ST2 in non-ST-elevation acute coronary syndrome in the MERLIN-TIMI 36 trial. Clin Chem 2012;58:257-66.
- Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation 2012;126:1596-604.
- Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004;110:3121-8.
- Gullestad L, Ueland T, Kjekshus J, et al. Galectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Eur Heart J 2012;33:2290-6.
- Lok DJ, Van Der Meer P, de la Porte PW, et al. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol 2010;99:323-8.
- van Kimmenade RR, Januzzi JL, Jr., Ellinor PT, et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol 2006;48:1217-24.
- Motiwala SR, Szymonifka J, Belcher A, et al. Serial measurement of galectin-3 in patients with chronic heart failure: results from the ProBNP Outpatient Tailored Chronic Heart Failure Therapy (PROTECT) study. Eur J Heart Fail 2013;15:1157-63.
- Fonarow GC, Adams KF, Jr., Abraham WT, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572-80.
- Manzano-Fernandez S, Januzzi JL, Jr., Boronat-Garcia M, et al. β-trace protein and cystatin C as predictors of long-term outcomes in patients with acute heart failure. J Am Coll Cardiol 2011;57:849-58.
- Nymo SH, Ueland T, Askevold ET, et al. The association between neutrophil gelatinase-associated lipocalin and clinical outcome in chronic heart failure: results from CORONA*. J Intern Med 2012;271:436-43.
- Macdonald S, Arendts G, Nagree Y, Xu X-F. Neutrophil Gelatinase-Associated Lipocalin (NGAL) predicts renal injury in acute decompensated cardiac failure: a prospective observational study. BMC Cardiovasc Disord 2012;12:8.
- Groenveld HF, Januzzi JL, Damman K, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol 2008;52:818-27.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239.
Keywords: Acute Coronary Syndrome, Acute Kidney Injury, Adrenomedullin, Aldosterone, Aminobutyrates, Amyloidosis, Anemia, Angiotensin II Type 2 Receptor Blockers, Angiotensin Receptor Antagonists, Arginine Vasopressin, Atrial Fibrillation, Blood Urea Nitrogen, Body Mass Index, Cardiomyopathy, Hypertrophic, Collagen, Comorbidity, Creatinine, Cystatin C, Dyspnea, Erythrocytes, Fatty Acid-Binding Proteins, Galectin 3, Glomerular Filtration Rate, Heart Failure, Hexosaminidases, Hospital Mortality, Hypertension, Pulmonary, Hypertrophy, Hyponatremia, Interleukin-18, Intramolecular Oxidoreductases, Lipocalins, Mineralocorticoid Receptor Antagonists, Myocardial Infarction, Myocarditis, Myocytes, Cardiac, Myofibroblasts, Natriuresis, Natriuretic Peptide, Brain, Neprilysin, Neutrophils, Obesity, Osmolar Concentration, Oxidative Stress, Peptide Fragments, Pericardium, Pharmaceutical Preparations, Pheochromocytoma, Protein Precursors, Pulmonary Embolism, Receptors, Peptide, Renin, Risk Factors, Stroke, Stroke Volume, Sympathetic Nervous System, Tetrazoles, Troponin, Troponin I, Troponin T, Valine, Vasodilation, Vasodilator Agents, Ventricular Dysfunction, Right, Ventricular Remodeling
< Back to Listings
lawsonlovervicieds.blogspot.com
Source: https://www.acc.org/latest-in-cardiology/articles/2015/02/09/13/00/cardiac-biomarkers-and-heart-failure
Belum ada Komentar untuk "Why Would We Continue to Draw Cardiac Biomarkers Every 8 Hours X 3"
Posting Komentar